Fotos / Sonidos
Autor
peptolabDescripción
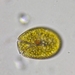
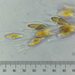
Caenomorpha medusula PERTY, 1852 from the superficial river edge benthos of the freshwater segment of the estuarine Peconic River. Imaged in Nomarsdki DIC on Olympus BH2S using Splanapo 40 0.95 and Splan 100 1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+.
This metopid ciliate is a fast swimmer and often difficult to track, but also holds on to the substrate thigmotactically with the dorsally located long cirri. It is an inhabitant of saprobic anoxic decaying benthic organic debris. My population averages 100 um in total length including the single long caudal spine. There are three macronuclear nodules with two sometimes incompletely separated and a single micronucleus. There is an anterior refractile granular agggregate. The cytoplasm contains diverse rod-shaped bacterial symbionts. The contractile vacuole was rather large in diastole in some individuals. All of these features are in agreement with the most recent redescription by Li et al 2017 (1).
Li et al 2017 provide the most recent and detailed redescription of the species. In describing the most important characters, they write: "Although this species has been studied for more than 150 years, species identification remains problematic because many descriptions are based only on live materials without redescription of the ciliature. Furthermore, some features, e.g. body shape, appear to vary among individuals in different environments, but, nonetheless, have traditionally been used as key characters in the taxonomy of Caenomorpha. The original description of Caenomorpha medusula by Perty (1852) was rather superficial and failed to note some important features (e.g. the numbers of adoral membranelles, bell kineties and spines, etc.), which renders the identification of this organism difficult. However, according to the original and subsequent investigations, this species can be recognized by a combination of the following characters: (i) multiple macronuclear nodules; (ii) two unequal-length bell kineties; (iii) one conspicuous posterior spine" (1).
"Description: Body medusoid, covered with a transparent rigid pellicle, 112–125 × 50–65 um in vivo with a ratio of length to width about 2:1. Posterior spine slender, about 45–55 um long in vivo; ratio of spine length to body length about 0.4. Plump rod-shaped epibiotic bacteria often found in US population but not found in China population. Cytoplasm clear and colourless, with some dark globules (1–2 m across) and an aggregate of transparent granules in anterior body part, rod-like bacteria exist in cytoplasm in China population, very likely endosymbionts. Edges of preoral bell never adjoin closely to posterior body; peristome narrow, deep funnel-shaped. Cytostome near base of spine; undulating membrane recognizable after staining, length of membrane about 50 m long. Contractile vacuole located near base of spine, about 15–20 m in diameter, pulsates at intervals of 3–5 min. Three (41 of 60 cells [Chinese population], 36 of 45 cells [US population]), four (19 of 60 cells [Chinese population], 8 of 45 cells [US population]) or five (1 of 45 cells [US population]) macronuclear nodules, usually ovoid or ellipsoidal, arranged in line, located in center of cell, sometimes incompletely separated; one micronucleus, ellipsoidal, near macronuclear nodules. Movement leisurely, spiraling while rotating around the long axis of the body" (1).
"Two strongly thigmotactic bell kineties about 68 um and 35 um long, respectively, located in anterior part of dorsal side of cell, consist of about 94 and 56 cirri (Chinese population), and 108 and 59 cirri (US population) respectively (n = 21); cirri in each kinety arranged in indistinct zig-zag pattern. Perizonal stripe beginning near anterior end of cell, about 6.4 um wide at middle part, composed of 114–180 kineties (114–169 in China population, 123–180 in US population), spiraling 450 degrees around axis; each kinety inclined about 60 degrees to edge of shield; longest kinety (at middle of stripe) composed of about 15 pairs of kinetosomes in both populations, whereas ones near oral region with only two pairs of kinetosomes. Adoral zone composed of 41-67 membranelles (Chinese population), each with three or four rows of kinetosomes, spiraling 360 degrees around body axis from near the distal perizonal stripe, terminates near cytostome. Undulating membrane on undersurface of preoral bell (i.e. roof of peristomial region), about 50 m long. Cilia on base of spine, invariably arranged in two short kineties, each 10–15 m long, composed of about 25 kinetosomes each. The two spine kineties inclined about 20 degrees to each other, converge posteriorly" (1).
Jankowski provided an older but elegant description: "Body medusoid, covered with a clear transparent rigid pellicle that looks like an armour; body length, without a spine, is 70-90 x 65-70 ul, spine 30-35 um long. The shield bears no ciliary meridians except for those of a perizonal ciliary stripe. Instead. it bears two groups of long thick flexible cirri, with 8-10 cirri in each group: they are perfectly seen from the left side. These cirri are highly thigmotactic- one can frequently observe a prolonged adhesion of the animals to sapropelic particles by the aid of these cirri, while both perizonal cilia and adoral membranelles continue their activity. The edges of the shield never adjoin closely to the body surface; instead, a narrow deep funnel may be observed between them. The perizonal ciliary stripe occupy the margin of the shield; it is composed of 5 ciliary rows not separated into two groups, unlike that of Metopidae (where it includes 2 upper and 3 lower kinetics). The PCS of Caenomorpha looks like a wide densely ciliated field composed by a number of short oblique ciliary rows with 5 kinetosomes each. It serves for both feeding and movement in this genus. The synchronous beating of the perizonal cilia and adoral membranelles produces a rotation of the swimming animal and, in addition, creates the intense water current along the buccal groove. driving the food-particles into the intrastomium. The cytostome in C. medusula occupies a typical for all the caenomorphids posterior position with a thin tubular cytopharynx raising right up into the anterior body part, where colourless food vacuoles are concentrated" (2).
- Description of two species of caenomorphid ciliates (Ciliophora, Armophorea): Morphology and molecular phylogeny. Song Li, William A. Bourland, Saleh A. Al-Farraj, Lifang Li and Xiaozhong Hu. European Journal of Protistology 61 (2017) 29–40.
- Morphology and Evolution of Ciliophora. III. Diagnoses and Phylogenesis of 53 Sapropelebionts, Mainly of the order Heterotrichida. ANATOL W. JANKOWSKI. Arch. Protistenk. Bd. 107, S. 185-294 (1964)
Fotos / Sonidos
Autor
polarblairxDescripción
Infesting my microscopy water, which sits on a desk next to a sunlit window
Fotos / Sonidos
Qué
Trachelius ovumAutor
peptolabDescripción
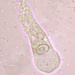
Trachelius ovum EHRENBERG, 1831 EHRENBERG, 1838 from the acidic freshwater kettle pond Chatfield's Hole. Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 20 0.75 and Splanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cells measure 240 um in length. The following discussions and several figures are adapted from Foissner et al (1995) (1).
According to EHRENBERG (1838), FOISSNER & FOISSNER (1988b) and KAHL (1931a), Trachelius ovum has several safe synonyms, which do not appear in the saprobiological literature: T. cicer SCHRANK- this older synonym was never used and should therefore be suppressed forever for reasons of stability, T. vorax EHRENBERG, Amphileptus rotundus MASKELL, Trachelius Leidyi FOULKE. The exact organization of this common ciliate was previously insufficiently known, although it was reported several times and has been processed using modern methods (DRAGESCO & DRAGESCO-KERNEIS 1986, FRYD VERSAVEL et al. 1975, SONG & WILBERT 1989). The representations are too schematized and incorrect in detail. We have therefore reworked T. ovum for the “Atlas”. This showed that the somatic and oral ciliation is very similar to that of Dileptus. Nevertheless, Trachelius is clearly demarcated from Dileptus namely by the ventro-lateral fossa, where the ciliation is slightly modified and the club-shaped mouth funnel, which consists of a thick layer of the finest fibrils.
Congener comparison: Trachelius subtilis PENARD, which has not yet been sufficiently confirmed, has only 12 contractile vacuoles and no suction cup. Dileptus species usually have a clearly pointed rear end and are always much slimmer. Paradileptus elephantinus lives predominantly in the pelagic of stagnant waters and has a rosary-shaped (moniliform) macronucleus. The characteristics are particularly important for identification are 2, 3, 4 .
Interestingly, I found one individual with two closely abutting oval macronuclei (see end of pictures after sampling site pictures). Trachelius subtilis PENARD, 1922, a species which Vďačný & Foissner (2) synonymized with T. ovum, is stated to have two oval macronuclear nodules. However, Martin Kreutz disagrees with Vďačný & Foissner and writes: "Trachelius subtilis was first described by Penard (1922). The species is smaller than Trachelius ovum and its main characteristic is a two-part macronucleus with a spherical micronucleus in the middle. In 2012 Vďačný & Foissner (2) synonymized Trachelius subtilis with Trachelius ovum with the argument that Penard (1922) possibly only found specimens of Trachelius ovum with a macronucleus constricted in the middle and that this is an observational error. Vďačný & Foissner obviously did not find any specimens with such a constricted macronucleus themselves. However, Penard was a very precise observer and Kahl (1935) also found specimens of Trachelius subtilis, which confirmed and supplemented Penard’s results" (3). Dr. Kreutz's examples do show two clearly separate macronuclear nodules with an intervening micronucleus in contrast to my observation of two closely abutting macronucleuar nodules with no visible micronucleus so I cannot rule out a folded dumbbell-shaped macronucleus appearing as two nodules in my example.
Differential diagnosis
Size in vivo 200-600 x 75-350 um, usually 250-350 um long.
Shape sac-shaped to almost spherical, starving specimens clearly flattened on one side. Proboscis often only about 1/4-, rarely up to 1/2-length, usually curved dorsally. In well fed specimens it becomes a short, stalk-shaped extension. Ventro-laterally a small, difficult to recognize pit that serves as a suction cup.
Macronucleus dumbbell-shaped, often disintegrates into a few spherical parts in postconjugates. Several micronuclei.
Many small contractile vacuoles scattered throughout the cell. Plasma very strongly vacuolated, the strands form a coarse network.
Short, rod-shaped extrusomes in the proboscis along the ridge of the mouth. Cortex thick, with many ellipsoid granules.
About 80-120 longitudinal rows of cilia, some of which extend into the ventro-lateral fossa, where there are several specializations, which are explained in the figure legends. Brush 3-4 rows, on the dorsal side of the trunk, a row extending almost to the end of the body; only clearly visible after silver impregnation and in the scanning electron microscope.
Mouth entrance at the base of the proboscis, surrounded by many very delicate bars that form a club-shaped, thick-walled funnel. To the right of the circumoral row of eyelashes there is a longitudinal row of cilia, on the left there are many short oblique rows of perioral cilia; more precise structure of the oral cilia can only be recognized after silver impregnation.
- FOISSNER W., BERGER H., BLATTERER H. & KOHMANN F. (1995): Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band IV: Gymnostomatea, Loxodes, Suctoria. – Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, 1: pp. 208-18
- VĎAČNÝ P. & FOISSNER W. (2012): Monograph of the dileptids (Protista, Ciliophora, Rhynchostomatia). – Denisia, 31: 1–529.
- https://realmicrolife.com/trachelius-subtilis/
Fotos / Sonidos
Autor
peptolabDescripción
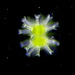
Paramecium bursaria Ehrenberg from the acidic freshwater kettle pond Chatfield's Hole. Imaged in Nomarski DIC on Olympus BH2S using SPlan 100 1.25 oil immersion and Splanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cells measure 120-146 um in length. This population is rather starved and most individuals have digested most of their Zoochlorella symbionts. I imaged one rotating well-fed free-swimming individual full of symbiont algae where we can see the three-dimensional relationship between macronucleus and micronucleus. I was able to closely image the contractile vacuoles which had collecting canals and 3 excretory pores. This is important to distinguish P. bursaria from a close congener, Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl 1935. Kreutz et al (2012) compared the two and found "Morphologically, P. chlorelligerum differs from P. (C.) bursaria, the second green species in the genus, by having a special swimming shape, the length of the caudal cilia, the size of the micronucleus, the size of the symbiotic algae, the contractile vacuoles (with collecting vesicles vs. collecting canals), and the number of excretory pores/contractile vacuole (1 vs. 2–3)" (1) .
PARAMECIUM BURSARIA Ehrenberg Length 90-150µm; foot-shaped, more or less flattened; uniform ciliation except for a group of long caudal cilia; green with symbiotic zoochlorellae; a long broad oral groove (vestibule) leads to the buccal cavity, the buccal ciliary apparatus is characterized by 2 " peniculi "; 1 macronucleus, 1 compact micro-nucleus; 2 contractile vacuoles; numerous prominent pellicular trichocysts. Diet-Food Bacteria (sometimes purple-coloured sulfur bacteria), flagellates; starving individuals digest their symbionts. Occurrence and ecology Cosmopolitan in distribution, e.g., Europe, North America, India, in still waters rich in higher plants and algae, such as ponds or littoral zones of lakes, forest pools rich in decaying leaves, oxidation ponds, polluted streams. Under laboratory conditions this ciliate occurs in cultures rich in peptone, under which conditions it can withstand high ammonium and low oxygen levels.
Paramecium bursaria is a mixotrophic ciliate species, which is common in stagnant and slow-flowing, nutrient-rich waters. It is usually found living in symbiosis with zoochlorellae (green algae) of the genera Chlorella or Micractinium. "Paramecium bursaria is one of only two species in the genus Paramecium that harbor algal endosymbionts. Based on a phylogenetic tree constructed from Paramecium 18S rRNA sequences with T. thermophila as outgroup, P. bursaria is the most diverged species since the most common Paramecium ancestor, which may explain why P. bursaria cell physiology is so distinct from other Paramecium species. In the wild, most P. bursaria cells stably harbor several hundred algal cells in the cytoplasm. Each algal cell is engulfed by a host-derived perialgal vacuole (PV) membrane that becomes attached to the host plasma membrane. Different species of algal endosymbionts have been identified in different strains of P. bursaria, suggesting that these strains may have evolved a preference for or compatibility with specific green algae" (2).
- Morphological and Molecular Characterization of Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl 1935 (Ciliophora) MARTIN KREUTZ, THORSTEN STOECK and WILHELM FOISSNER. J. Eukaryot. Microbiol., 59(6), 2012 pp. 548–563
- Genome plasticity in Paramecium bursaria revealed by population genomics. Yu-Hsuan Cheng, Chien-Fu Jeff Liu, Yen-Hsin Yu, Yu-Ting Jhou, Masahiro Fujishima, Isheng Jason Tsai & Jun-Yi Leu. BMC Biology volume 18, Article number: 180 (2020)
Fotos / Sonidos
Autor
crseaquistDescripción
Gathered dry leaves on 2024-02-23 and stored in water.
Here are some videos:
https://youtu.be/8DMTa0ZruLc
https://youtu.be/izC2XI3pRPY
https://youtu.be/1Q_9wdLj5pc
I think the videos seem to show it is an eruptive morphotype.
Fotos / Sonidos
Autor
mnold1Descripción
Mag. 400x
ID based on comparison with observations here on iNat. While observing this specimen I noticed the apparently attached vesicle at the posterior end. The following photos and linked video document the predation or autolysis of the cell. Which is it?
video link: https://youtu.be/O7FWG9cptek
- A water sample was taken on 11/08/2022, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 55°F.
Qué
Hongos Ascomicetes Y Líquenes (Subfilo Pezizomycotina)Autor
mlankfordDescripción
Fresh water pond
Fotos / Sonidos
Autor
mnold1Descripción
Mag. 400x
Spicules (structural component) of freshwater sponges, as seen here https://ars.els-cdn.com/content/image/3-s2.0-B9780123748553000042-gr16.jpg.
- A pond edge water sample (freshwater) was taken on 2/9/2022 using a 10 micron dip net to enrich for microorganisms. Air temp. 41C. Sampling area was free of ice due the water flow through a nearby culvert.
Fotos / Sonidos
Autor
mnold1Lugar
Barn Island Wildlife Management Area - 249 Palmer Neck Rd, Pawcatuck, CT 06379, USA (Google, OSM)Descripción
Mag. 400x
As seen here https://inpn.mnhn.fr/espece/cd_nom/829371/tab/fiche and http://www.harmfulalgalblooms.ir/en/index.php/habs-encyclopedia/21-taxonomic-description/eukaryota/division-chromophyta/class-bacillariophyceae/biddulphiales/biddulphiineae/136-odontella-mobiliensis-bailey-grunow.html. Previously assigned to 4 different genera https://www.algaebase.org/search/species/detail/?species_id=148450&-session=abv4:AC1F0C4D1b5312793CVS68F30225
From one of the main tidal channels of the salt marsh, a water sample was taken on 11/10/2021 using a 10 micron dip net to enrich for microorganisms.
Fotos / Sonidos
Qué
Género UronychiaAutor
mnold1Lugar
Barn Island Wildlife Management Area - 249 Palmer Neck Rd, Pawcatuck, CT 06379, USA (Google, OSM)Descripción
Mag. 400x
As seen here https://d3i71xaburhd42.cloudfront.net/c5a1a543a7bc6288de4a3db3969aba6979e984a1/8-Figure4-1.png
From one of the main tidal channels of the salt marsh, a water sample was taken on 11/10/2021 using a 10 micron dip net to enrich for microorganisms.