Qué
Perico Dorsirrojo (Psephotus haematonotus)Autor
george_seagullDescripción
The exact moment I was noticed. Northern Memorial Park, Glenroy.
Fotos / Sonidos
Qué
Búho Cornudo (Bubo virginianus)Autor
eric_houghDescripción
Heard hooting, seen in photo perched on saguaro during Aurora borealis that came through much farther south than perhaps ever before.
Qué
Ciempiés Cobrizo (Lithobius forficatus)Autor
kavaliauskasDescripción
www.dantis.net/x3/
Lithobius forficatus, akmenlindÄ
Lithuania, TauragÄ, 2021.08.15
Canon EOS R
Canon MP-E 65mm f/2.8 1-5x Macro
Fotos / Sonidos
Autor
kendrasveaDescripción
I believe this may be a flicker - found in our chimney.
Fotos / Sonidos
Qué
Carpintero Bellotero (Melanerpes formicivorus)Autor
anudibranchmomDescripción
One of TWO (!) leucistic individuals seen by many. This is the "darker" one, with a bit more black, and very nice eyeliner :-) Probably in the same family group; normally colored individuals in the group as well.
Fotos / Sonidos
Autor
hsug1747Descripción
Shot through the world’s smallest fisheye lens.
2 image stack (handheld) processed with https://focusstackingonline.com , retouched in PS express
Fotos / Sonidos
Qué
Hydra oxycnidaAutor
closterium_mysteriumDescripción
This is an observation of several Hydra specimens taken from a single water sample. All images with 40x and 100x lenses are taken from the same specimen.
A water sample was taken from the shore of lake Istok. The sample was stored at room temperature and observed 6 days after collection.
Video: https://youtu.be/j2zLH1NoOc4
Fotos / Sonidos
Autor
mhinczDescripción
Collected from freshwater sample, 100x magnification. Identification based on key found here: https://www.caryinstitute.org/sites/default/files/public/reprints/thorp_covich_gastrotrichs_2010.pdf
Fotos / Sonidos
Autor
kenkDescripción
Specimen approximately 2mm. On the shell of a California Mussel (Mytilus californianus). The GIF is at 50% speed.
Fotos / Sonidos
Autor
closterium_mysteriumDescripción
Fig. 1-3: Valve view
Fig. 4-6: Girdle view
GIF and Fig. 7-9: A few images as the diatom rotates
A water sample was taken from the bank of the Vuoksi River. The sample was stored at room temperature and observed one day after collection.
Video: https://youtu.be/n7HxWvJmyp4
Observation of Entomoneis ornata https://www.inaturalist.org/observations/192091414
Qué
Murciélago Cola Suelta Mexicano (Tadarida brasiliensis)Autor
dave_pgDescripción
Moon at upper left. Cropped one second exposure with low intensity flash every 20 milliseconds, 52mm, f2.8, ISO 1000. Distance 30-40 feet.
Qué
Gallo Y Gallina Domésticos (Gallus gallus var. domesticus)Autor
djlavorLugar
Falta ubicaciónDescripción
Feral chicken. This species is native to southeast Asia but has feral populations throughout Guam.
Fotos / Sonidos
Qué
Puma (Puma concolor)Autor
billhubickDescripción
I was listening for owls not too long after dusk. The temperature was in the 50s and the half moon shone brightly. Owls had been vocal. Standing in the dark, I heard a very loud, unfamiliar call that I thought must be either Great Horned or Spotted Owl. It called again, nearby and more intensely. I found myself getting in my car quickly without quite knowing why - it was an involuntary response faster than my brain consciously moved on to IDs like this one. But my brain did get there as I found myself rolling up the window in addition to closing the door, leaving just enough room to record. It was too loud and intense. The sounds moved very quickly from roughly behind my car to in front of my car. When I turned on the car and drove slowly forward, I quickly spotted these two individuals (presumably immature), which I somehow half expected I would see up the road. They were occasionally responding to other calls from the woods (presumably their mother). Photo taken by headlights at 12800 ISO.
Fotos / Sonidos
Autor
lamawebberDescripción
Thalassiosira pacifica Gran & Angst, 1931
Phylum: Bacillariophycophyta, Subphylum: Bacillariophytina, Class: Mediophyceae, Order: Thalassiosirales, Family: Thalassiosiraceae.
SEM images of the marine diatom Thalassiosira pacifica Gran & Angst, 1931.
Girdle view: Cells 16.1-28.8 µm (7.0–55.0) µm in diameter. GI = n = 3.
Cells rectangular with rounded low mantle. Valve face flat or slightly concave. Connecting thread about as long as the pervalvar axis.
Valve view:
Loculate areolae in linear (straight), eccentric or fasciculate patterns (curved) rows depending on the diameter of the cell. Central process (A.) adjacent to a central areola (annulus) (B.). Valve face areolae (C.) 10-19 in µm (Hasle and Syvertsen 1996, Li et al. 2014). GI = 14-16 in 10 µm. 20 to 28 areolae in 10 µm (Hoppenrath et al. 2007, not so). Valve face covered by tiny siliceous granules (D.) (Hoppenrath et al. 2007). Marginal strutted processes (MSPs) with relatively long and coarse external tubes. GI specimens have a bulbous and coarse flared tip. MSPs 4-7 in 10 µm. GI = 4-6 in 10 µm. Separation between MSPs = 1-2 µm (GI) Mantle areolae smaller than those on valve face. The height of mantle is about 2–3 areolae (Li et al. 2014). Labiate process positioned as for a marginal strutted process, slightly inside the marginal ring. Labiate process slightly larger than the marginal strutted processes.Valve margin ribbed.
Notes: Mantle areolae smaller than those on valve face. Valve margin ribbed. Distinguished from T. angulata by the ribbed margin and the more closely spaced marginal processes with shorter
external tubes and the location of the labiate process. (Hasle and Syvertsen 1996:37; Mahood et al. 1986; Hoppenrath et al. 2007; Li et al. 2013. (Hoppenrath et al. 2007 –Fig. 45-46, scale bar = 10 µm)
Methods:
A distal section of the eelgrass Zostera marina was heated and digested in 95-99C sulfuric acid, rinsed in distilled water and further cleaned or organics with 30% hydrogen peroxide and with further rinsing, brought to a pH of 6.5. Pipetted and syringed with further washes of sterile distilled and deionized water using a Swinnex filter holder onto 12 mm Teflon 0.2 µm filters and attached to 13 mm SEM stub with double sided tape. Imaging with a Hitachi s4800 SEM at the AMF at UVIC. Thank you Ron Read for taking the SEM images and Elaine Humphrey for SEM support at University of Victoria, BC, Canada. Adjusted in Photoshop.
References:
Cupp, E. E. 1943. Marine Plankton Diatoms of the West Coast of North America. University of California Press. Berkeley, California.
Gran, H.H. and Angst, E.C. (1931). Planktonic Diatoms of Puget Sound. Seattle, University Press. https://babel.hathitrust.org/cgi/pt?id=uc1.31822010334225&view=1up&seq=3
Hoppenrath, M., Beszteri, B., Drebes, G., Halliger, H., Van Beusekom, J.E.E. , Janisch, S. and Wiltshire, K. H. (2007) Thalassiosira species (Bacillariophyceae, Thalassiosirales) in the North Sea at Helgoland (German Bight) and Sylt (North Frisian Wadden Sea) – a first approach to assessing diversity, European Journal of Phycology, 42:3, 271-288, DOI: 10.1080/09670260701352288
Horner, R.A., Postel, J.R., Halsband-Lenk, C., Pierson, J.J., Pohnert, G. and Wichard, T. 2005.Winter-spring phytoplankton blooms in Dabob Bay, Washington. Prog. Oceanogr. 67 (3-4): 286-313.
Hasle, G.R. & Syvertsen, E.E. (1996). Marine Diatoms. In: Identifying Marine Phytoplankton. (Tomas, C.R. Eds). San Diego: Academic Press.
Guiry, M.D. & Guiry, G.M. 2007, AlgaeBase version 4.2. World-wide electronic publication, National University of Ireland, http://algaebase.org, searched April 10, 2022.
Li, Y. and Lu, S. (2013). The genus Thalassiosira off the Guangdong coast, South China Sea. Botanica Marina; 56(1): 83–110. DOI: 10.1515/bot-2011-0045
Mahood, A.D., Fryxell, G.A., & Mcmillan, M. (1986). The diatom genus Thalassiosira: species from the San Francisco Bay system. Proc. Calif. Acad. Sci. 24: 127–156.
Round, F.E., Crawford, R.M. and Mann, D.G. (1990). The Diatoms, Biology & Morphology of the Genera. Cambridge University Press, Cambridge, UK. pp. 132-133.
Hay, M. B., Pienitz, R., and Thomson, R.E. (2003). Distribution of diatom surface sediment assemblages within Effingham Inlet, a temperate fjord on the west coast of Vancouver Island (Canada). , 48(3-4), 291–320. doi:10.1016/s0377-8398(03)00025-2
Sancetta, C. and Calvert S. E. (1988). The annual cycle of sedimentation in Saanich Inlet, British Columbia:implications for the interpretation of diatom fossil assemblages. Deep-Sea Research, Vol. 35, No. 1, pp. 71-90.
Shim, J. H. (1976). Distribution and Taxonomy of Planktonic Marine Diatoms in the Strait of Georgia, B.C. Phd. Thesis, UBC.
Waters, R. E., Brown, L.N., and MG Robinson, M.G. (1992). Phytoplankton of Esquimalt Lagoon, British Columbia: comparison with west Vancouver Island coastal and offshore waters. Canadian Technical Report of Hydrography Ocean Sciences 137.
Qué
Ganso Canadiense Mayor (Branta canadensis)Autor
that_bug_guyDescripción
Descending from the sky was a flock of Canada Geese. They entered the water with a splash!
One of them began to dunk its head underwater, bringing it back up to cover its body in water. It was bathing! A few other geese followed suit. They did so in rapid succession.
You can really see how water-resistant a gooses's feathers are.
Fotos / Sonidos
Qué
Sapo Dorado (Incilius periglenes)Autor
zacpetersonDescripción
Date is approximate. 3 individuals (2 males & 1 female) seen sometime in April, 1987. My mother and father (@dmpeterson ) were living in Monteverde in the spring of 1987, staying with the Gavin family. I have been digitizing their old slides, and thought these ones were significant, so I uploaded them. The date and location are approximate, based on their best recollection.
Qué
Murciélago Perro Mayor (Peropteryx kappleri)Autor
svaldvardDescripción
Peropteryx kappleri
Estado de Amazonas
Brasil
Qué
Phidippus clarusAutor
sdroegeDescripción
Jumping spiders are so darn cute. Its all about the eyes, you get to see 4 of the 8. Great pets too, This one (Phidippus clarus) was found at the MAGLEV site for their proposed Rail Yard on public lands. Good by Phidippus if that happens. Although, they can be pretty touch so maybe some would be happy hunting flies in concrete and gravel. Collected by Danie Phan and photoed by Anders Croft.
~~~~~~~~~~{{{{{{0}}}}}}~~~~~~~~~~
All photographs are public domain, feel free to download and use as you wish.
Photography Information:
Canon Mark II 5D, Zerene Stacker, Stackshot Sled, 65mm Canon MP-E 1-5X macro lens, Twin Macro Flash in Styrofoam Cooler, F5.0, ISO 100, Shutter Speed 200
We Are Made One with What We Touch and See
We are resolved into the supreme air,
We are made one with what we touch and see,
With our heart's blood each crimson sun is fair,
With our young lives each spring impassioned tree
Flames into green, the wildest beasts that range
The moor our kinsmen are, all life is one, and all is change.
- Oscar Wilde
You can also follow us on Instagram - account = USGSBIML
Want some Useful Links to the Techniques We Use? Well now here you go Citizen:
Best over all technical resource for photo stacking:
www.extreme-macro.co.uk/
Free Field Guide to Bee Genera of Maryland:
bio2.elmira.edu/fieldbio/beesofmarylandbookversion1.pdf
Basic USGSBIML set up:
www.youtube.com/watch?v=S-_yvIsucOY
USGSBIML Photoshopping Technique: Note that we now have added using the burn tool at 50% opacity set to shadows to clean up the halos that bleed into the black background from "hot" color sections of the picture.
www.youtube.com/watch?v=Bdmx_8zqvN4
Bees of Maryland Organized by Taxa with information on each Genus
www.flickr.com/photos/usgsbiml/collections
PDF of Basic USGSBIML Photography Set Up:
ftp://ftpext.usgs.gov/pub/er/md/laurel/Droege/How%20to%20Take%20MacroPhotographs%20of%20Insects%20BIML%20Lab2.pdf
Google Hangout Demonstration of Techniques:
plus.google.com/events/c5569losvskrv2nu606ltof8odo
or
www.youtube.com/watch?v=4c15neFttoU
Excellent Technical Form on Stacking:
www.photomacrography.net/
Contact information:
Sam Droege
sdroege@usgs.gov
301 497 5840
Autor
airgelDescripción
Peculiar behaviour. Got a few quick photos before they (presumably) woke up and flew off.
Fotos / Sonidos
Autor
flowntheloopDescripción
Parasitic fungus on a fungus gnat (Sciara sp.) I think?
Fotos / Sonidos
Qué
Tlacuache Norteño (Didelphis virginiana)Autor
cowturtleDescripción
365nm blacklight
Qué
Paloma Doméstica (Columba livia var. domestica)Autor
george_seagullDescripción
Shopping for salt.
Fotos / Sonidos
Qué
Araña Cangrejo de Banda Blanca (Misumenoides formosipes)Autor
tshahanFecha
Septiembre 2022Fotos / Sonidos
Qué
Avispa Chaqueta Amarilla Occidental (Vespula pensylvanica)Autor
thumbwaveDescripción
Tearing off the abdomen of a bee caught by a California Bee Assassin (Apiomerus californicus).
REF: https://www.inaturalist.org/observations/130029872
Timeline:
09:46:14 bee captured by Bee Assassin
09:48:12 Yellowjacket is latched on to bee
09:49:40 Yellowjacket has separated bee abdomen from thorax
Fotos / Sonidos
Qué
Lombrices de Aguas Negras (Género Tubifex)Autor
augochloraDescripción
A cluster of tubifex worms.
Fotos / Sonidos
Autor
lamawebberDescripción
Coscinodiscus radiatus Ehrenberg 1840
Class: Coscinodiscophyceae, Order: Coscinodiscales, Family: Coscinodiscaceae, Genus: Coscinodiscus. Image of Coscinodiscus radiatus Ehrenberg 1840. Cells isolated on December 15, 2009 from Trincomali Channel (TC), Spanish Hills Wharf, Galiano Island, B.C.
A common coastal marine diatom, not reported from polar regions. Cells are solitary. Not abundant, but common throughout the year in our TC samples. Cells flat (coin shaped) or only slightly convex with a shallow mantle and round edge (margin) [Images 1-3]. Diameter range reported as: 13-218 µm (Hasle et al., 1986, Sancetta, 1987, Sar et al., 2010). The plastids are numerous and cocciform. Central rosette of areolae (pores), that may be indistinct, and with a hyaline area ranging from apparent to indistinct. Hyaline lines are absent (Hoppenrath et al., 2009) [Images 1, 4]. The range of areolae in 10 µm is 3.5-6 near the centre, 2-6 in the middle of the valve, and 4-8 at the valve margin [Image 1, 4]. Small rimoportulae are randomly distributed throughout the valve; they are considerably smaller than the valve areolae [Images 1, 4]. Cribra are slightly domed and covers the areolae [Images 1-3]. One ring of marginal rimoportulae [Images 1-2]: there are two macrorimoportulae with a reported angle range of between 129-145 degrees and numerous microrimoportulae 2-4 per 10 µm. Three bands comprise the cingulum [Images 1-2]. One band has numerous small areolae along striae located on the pervalvar axis [Image 4] reported at 45-50 in 10 µm (Hasle et al., 1996, Sar et al., 2010).
Observations on Trincomali Channel samples and notes on diagnostic features:
Areolae: A central rosette of areolae (pores), that may be indistinct, and with a hyaline area ranging from apparent to indistinct. Hyaline lines are absent (Hoppenrath et al., 2009). The areolae are large for this genus—averaging 0.8 µm in diameter for the TC isolates [Images 1, 4], and generally consistent in diameter with two exceptions; 1) much smaller at the margin (Al-Yamani et al., 2009), and 2) for small cells (Sar et al., 2010). Areolae form in radial rows of decussating (intersecting) arcs. The reported range of areolae in 10 µm is 3.5-6 near the centre, 2-6 in the middle of the valve, and 4-8 at the valve margin (Hasle et al., 1986, Hasle et al., 1996, Sar et al., 2010). This matches the measurements for this isolate: 5-5.5 in 10 µm near the centre, 5-6 in the middle of the valve and 5-6 µm in 10 µm at the valve margin (equivalent to the 2009 TC isolate and clone) [Images 1, 4].
Single opening in the hyaline area: A small opening has been reported on the hyaline area of the exterior valve that does not reach into the interior (Hasle et al. 1986); not reported in Sar et al. (2010), found in one deeply cleaned 2011 TC isolate (Images 19-20); not yet found in our 2019 TC isolate and cleaned clones.
Cribra: Cribra are slightly domed and covers the areolae [Images 1-3]. The areolae have numerous small pores. Pore measurements for other TC isolates derived from SEM images range from 0.4 µm to 1 µm. Unlike C. perforatus—a species easily confused with C. radiatus (Sar et al., 2010)—C. radiatus has no subsidiary perforations around the cribrum; however, in C. radiatus there are noticeably larger poroids circling around the outside of the cribra. The 2019 TC isolate has cribra filled with a range of 86 to 98 pores: Hasle et al. (1986) has images of cribra showing a range of between 54 and 149 (our counts). Hasle et al. (1986) caution on using differences in cribra structure for Coscinodiscus identifications. However, Sar et al. (2010) consider the differences in cribra pattern important, at least for distinguishing C. radiatus from C. perforatus. These differences in Coscinodiscus species require further investigation.
Valve rimoportulae: Scattered across the valve are small rimoportulae, much smaller than the areolae, that may differ in quantity amongst cells and by size of cells (Sar et al., 2010) [Image 1]. Valve rimoportulae in the TC 2011 and 2019 isolates measured 0.3 µm in diameter and exhibit the same variability in distribution noted by Sar et al. (2010).
Marginal rimoportulae: In girdle view, the cells are distinctively coin shaped (discoid) with a narrow perivalvar axis (Hasle et al., 1996, Sar et al., 2010) with one ring of marginal rimoportulae [Images 1-3]. The marginal ring has two macrorimoportulae with a reported angle of between 129-145o (Sar et al., 2010); clone J (2019) has a range of between 124 -137 degrees. By imaging with SEM, the inner macrorimoportulae are seen to be long and tubular with slit like openings. The outer opening is indented on the mantle and is visible with LM. There are regularly spaced smaller microrimoportulae along the marginal ring also visible with a LM, 2-4 per 10 µm Image 1, and for this 2009 isolate, 3-4 per 10 µm [Image 1]. Though they have a similar anvil shape, these microrimoportulae are noticeably shorter than the macrorimoportulae (Hasle et al., 1996). For Clone J-2019, the former are 0.55 µm and the latter are 0.9 µm at their widest dimension. The slits of both types of rimoportulae face the same direction, away from the valve centre, though we have occasionally observed microrimoportulae turned in dissimilar orientations. Coscinodiscus radiatus rimoportulae are morphologically different and larger than for C. perforatus, including a larger external opening of the macrorimoportulae (visible with LM) (Sar et al., 2010). Macro and micro rimoportulae are a diagnostic feature for Coscinodiscus species (Hasle et al. 1986).
Cingulum: There are three bands comprising the cingulum (Hasle et al., 1986, Sar et al., 2010) [Images 1-2]. One band has numerous fine areolae along striae located on the pervalvar axis [Image 4] reported at 45-50 in 10 µm (Sar et al., 2010); the 2011 and 2019 TC isolates have 40-43 in 10 µm.
Methods: C. radiatus cells isolated by micro-pipette from a general plankton tow with a 60 micron net at Spanish Hills Wharf, Trincomali Channel, North Galiano Island (N48˚ 59.688, W123˚ 35.064), Southern Gulf Islands, British Columbia, Canada, December 15, 2009. Cells isolated by micro-pipette and cleaned in at least three drops of sterile TC water. Transferred into HESNW growth media provided by the Canadian Center for the Culture of Microorganisms (CCCM, culture #: SHW-Cg-01) at the University of British Columbia, Vancouver. For SEM, cells preserved with 2% Formalin and washed repeatedly with distilled water through 13 mm plastic filters to remove salts (see complete method used in iNaturalist, Galiano Island, Attheya longicornis). Filters dried and stuck onto aluminium SEM stubs. Stubs sputter coated with gold. The SEM images were taken with a Hitachi S-4700 FESEM at the UBC Bioimaging Facility, University of British Columbia, Vancouver. Many thanks to Elaine Humphrey and Derrick Horne who assisted in sample preparation and the taking the SEM images.
Images:
Image 1: SEM image. Valve view showing flat circular shaped cell. Isolated on December 15, 2009 into f/10 growth media and cloned. Gold sputter coated. SEM image taken with a Hitachi 4700 at the UBC Bioimaging Facility.
Image 1b: SEM image. Valve view showing flat circular shaped cell. Isolated on December 15, 2009 into f/10 growth media and cloned. Gold sputter coated. SEM image taken with a Hitachi 4700 at the UBC Bioimaging Facility.
Image 2: SEM (Scanning Electron Microscope) image. Valve view showing flat, coin shaped cells. Isolated on December 15, 2009 into f/10 growth media and cloned. Gold sputter coated. SEM image taken with a Hitachi 4700 at the UBC Bioimaging Facility.
Image 3: SEM (Scanning Electron Microscope) image. Valve view showing flat, coin shaped cells. Isolated on December 15, 2009 into f/10 growth media and cloned. Gold sputter coated. SEM image taken with a Hitachi 4700 at the UBC Bioimaging Facility.
Image 4: SEM (Scanning Electron Microscope) image. Inner valve view showing flat, coin shaped frustule. Isolated on December 15, 2009 into f/10 growth media and cloned. Gold sputter coated. SEM image taken with a Hitachi 4700 at the UBC Bioimaging Facility.
References:
Al-Yamani, F.Y. & Suburova, M.A. (2019). Marine phytoplankton of Kuwait's waters Volume II. Diatoms. pp. [1]-336, 161 pls. Kuwait: Kuwait Institute for Scientific Research.
Bérard-Therriault, L., Poulin, M. & Bossé, L. (1999). Guide d'identification du phytoplancton marin de l'estuaire et du Golfe du Saint-Laurent incluant également certains protozoaires. Publication Spéciale Canadienne des Sciences Halieutiques et Aquatiques 128: 1-387. pp. 30-31, p.85. plate 14 a-c.
Cupp, E. E. (1943). Marine Plankton Diatoms of the West Coast of North America. Bull. Scrips. Inst. Oceanography. 5: 1-238. p. 56. Fig. 20.
Guiry, M.D. in Guiry, M.D. & Guiry, G.M. 2020. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 20 January 2020.
Hasle, G.R. & Syvertsen, E.E. (1996). Marine diatoms. In: Identifying Marine Phytoplankton. (Tomas, C.R. Eds), pp. 107-109. San Diego: Academic Press.
Hasle, G. R., & Sims, P. A. (1986). The Diatom Genus Coscinodiscus Ehrenb.: C. argus Ehrenb. and C. radiatus Ehrenb. Botanica Marina, 29(4).
Hoppenrath, M., Elbrächter, M. & Drebes, G. (2009). Marine phytoplankton Selected microphytoplankton species from the North Sea around Helgoland and Sylt. pp. [1]-264, figs 1-87. p. 32, fig. 12d-g. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung.
Horner, R. (2002). A Taxonomic Guide to Some Common Marine Phytoplankton. Biopress Ltd. Bristol, UK.
Phyto'pedia - The Phytoplankton Encyclopaedia Project, UBC Department of Earth, Ocean and Atmospheric Sciences: https://www.eoas.ubc.ca/research/phytoplankton/diatoms/centric/ditylum/d_brightwellii.html Accessed January 17, 2020.
Rao, V.N.R. and Levin, J. (1976). Benthic marine diatom flora of False Bay, San Juan Island, Washington. Syesis, 9:173–213.
Round, F.E.,Crawford, R.M. & Mann, D.G. (1990), The Diatoms, Biology & Morphology of the Genera, pp. 176-177. Cambridge University Press, Cambridge, UK.
Sancetta, C. (1987). Three Species of Coscinodiscus Ehrenberg from North Pacific Sediments Examined in the Light and Scanning Electron Microscopes. Micropaleontology, Vol. 33, No. 3. pp. 230-241.
Sar, E. A., Sunesan, I. & Jahn, R. (2010). Coscinodiscus perforatus revisited and compared with Coscinodiscus radiatus (Bacillariophyceae). Phycologia 49(6): 514-524.
Shim, J. H. (1976). Distribution and Taxonomy of Planktonic Marine Diatoms in the Strait of Georgia, B.C. Phd. Thesis, UBC. P. 139. P. 233 Plate XII. 12.
Tynni, R. (1986). Observations of diatoms on the coast of the state of Washington. Geological Survey of Finland, Report of Investigation 75. P. 12. Plate V-27.
Waters, RE, LN Brown, and MG Robinson. (1992). Phytoplankton of Esquimalt Lagoon, British Columbia: comparison with west Vancouver Island coastal and offshore waters. Canadian Technical Report of Hydrography Ocean Sciences 137.
Webber, M. and Humphrey, E. (2020). A Quick and Simple Technique for Orientating Diatoms for SEM and Light Microscopy. Microscopy Today. 28(1), 30-33.
Fotos / Sonidos
Qué
Oligoquetos (Subclase Oligochaeta)Autor
animalculeDescripción
All different sized. In my freshwater tank that I dump samples in, so I have no clue where it could have come from. The longest ones are about 6 in long. And they’re all holographic…I first spotted them on the night of the Full Worm Moon, and it seems as though they all come to the sides of the tank and “swarm” when the moon is full or new…so we’ve dubbed them Moon Worms!
Fotos / Sonidos
Qué
Rata Gris Asiática (Rattus norvegicus)Autor
gabrivaudaDescripción
2 esemplari osservati
Fotos / Sonidos
Qué
Rana Manchada Tarareante (Chiasmocleis albopunctata)Autor
anudibranchmomDescripción
ID tentative, possibly another species in this genus.
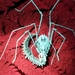
Possibly my favorite “Two Species” observation from the trip! The large tarantula clearly had a commensal relationship with the little 2cm frog, which mostly stayed hidden underneath the tarantula. They shared a burrow. When the frog emerged from underneath the tarantula briefly, the tarantula slowly extended a gentle leg (first photo) and guided the frog back underneath its body to safety. 😍😍😍😍😍
I found several papers written on this amazing subject but never expected to see it for myself in the wild. Incredible experience!!
Qué
Termitas (Epifamilia Termitoidae)Autor
anudibranchmomDescripción
Mounds looked like a cemetery in this pasture!
Fotos / Sonidos
Autor
aucklandmuseumDescripción
Austrovenus aucklandica
Holotype
Collected: 1922
Collected by: J. Bollons
https://www.aucklandmuseum.com/collections-research/collections/record/am_naturalsciences-object-197646
Fotos / Sonidos
Qué
Cascabel Cornuda del Noroeste (Crotalus cerastes)Autor
finaticDescripción
Audio recorded on cellphone. Trimmed and amplified using Audacity. It is one buzz repeated a second time.
Qué
Fragata Pelágica (Fregata minor)Autor
gcmpDescripción
Great Frigatebird (Fregata minor) arriving at nest, San Benedicto Island, Revillagigedo Archipelago Biosphere Reserve (Socorro Islands), Pacific Ocean, Western Mexico, December
Fotos / Sonidos
Qué
Paloma Doméstica (Columba livia var. domestica)Autor
george_seagullDescripción
A series of disturbing images, and a reminder to always wash your vegies.
Qué
Labidura ripariaAutor
pufferchungDescripción
UVIVF
I didn't have a chance to take a picture with regular light. It ran away while I was changing light source.
Fotos / Sonidos
Qué
Metorito de Pino (Microtus pinetorum)Autor
nflicker101Descripción
Don't worry I handled it gently. Attacked by my cat, I saved it and relocated it. Unhurt except it lost a little fur on its head.
Fotos / Sonidos
Qué
Phanaeus vindexAutor
tshahanDescripción
found in (and returned to) soil while gardening
Fotos / Sonidos
Qué
Ganso Egipcio (Alopochen aegyptiaca)Autor
robinia396Descripción
Parents with 2 beautiful chicks.
Fotos / Sonidos
Autor
bushboyDescripción
She's STILL not forgiven me for once stroking her nymphal antennae!
I think Spike's a girl - fat tum & foul temper. All bluff, unlike her bigger cousins, Polyspilota aeruginosa, she never tried to stab me with her fearsome forelimbs and certainly didn't draw blood. Released quickly back in her thicket where she gradually calmed (last pic).
See her development from final nymphal instar. Here: https://www.inaturalist.org/observations/16163662
And here: https://www.inaturalist.org/observations/16442575
Fotos / Sonidos
Autor
jane_trembathDescripción
Fine cilia on its body set up a powerful vortex drawing food to its mouth. It regularly contracted its front end, maybe swallowing food.
Fotos / Sonidos
Qué
Rascador Moteado (Pipilo maculatus)Autor
beartrackerDescripción
calls. This is a different call than the hoarse call normally made. I photographed the bird as it was making this call.
Fotos / Sonidos
Qué
Rana Toro (Lithobates catesbeianus)Autor
tysmithDescripción
Defense posturing and calling. Consider the audio, yes it is actual audio, a try not to laugh challenge.
Qué
Foca de Weddell (Leptonychotes weddellii)Autor
pczikoDescripción
Underwater recording of Weddell Seals by the McMurdo Oceanographic Observatory (www.moo-antarctica.net). Of potentially soniferous marine mammals, only Weddell seals occur in the area at this time of year. Verified also by regular visual/video observation. Hydrophone at 21m deep. (Attached photo is example of Weddell seal near the observatory site, but was not taken at the same time as the audio recording).